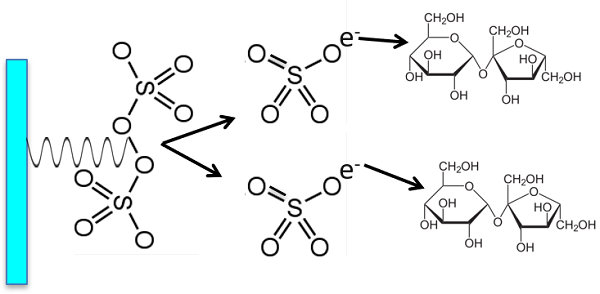
The One Reagent contains both acid and persulphate – why doesn’t it start to oxidize the organics?
Persulphate does not oxidize the organics until the UV light is turned on. The UV reacts with the persulfate to form sulfate radicals which also oxidize organic compounds to CO2.