PCR Reaction Setup and AMPure XP Application
Reproducible and Efficient Automation of PCR Reaction Setup and Purification Using the Biomek 4000 Workstation and AMPure XP
Ruth Zhang, Li Liu, Amy Yoder, John Palys, Bryan Daniels, Isabel Gautreau and William Godfrey
Beckman Coulter Life Sciences, Indianapolis, IN
Abstract
The Biomek 4000 Laboratory Automation Workstation has been used to demonstrate highly reproducible real-time amplification (CV<2%) after automated reaction setup. In addition, the Agencourt AMPure XP reagent system has been automated on the Biomek 4000 Workstation to provide rapid amplicon purification (two 96-well plates in less than 90 minutes) with yield and purity equivalent to manual purification. Both procedures were demonstrated to be free of cross-contamination.
Introduction
Polymerase chain reaction (PCR) is a fundamental and essential technique for medical and biological research, as well as forensic identification. Its exceptional ability to accurately amplify and quantify extremely low levels of nucleic acids has driven the emergence of numerous applications, including genetic testing, tissue typing, infectious disease identification, genetic fingerprinting, DNA sequencing and cloning, and gene expression. It is now used in a wide range of formats, including RT-PCR, qPCR, Multiplex PCR, Inverse PCR, digital PCR, and Miniprimer PCR.
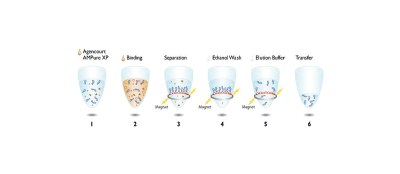
Figure 1. Schematic representation of the workflow for the six-step automated amplicon purification procedure.
Given the number of samples analyzed by PCR, the multitude of amplification formats used, and the need to reduce assay costs, a simple, easy-to-use, and high-throughput automation solution for PCR reaction setup is absolutely necessary. Automation of reaction setup can also eliminate both human error and contamination associated with manual processing, while assuring high quality results.
Efficient recovery of pure PCR amplicons is essential for downstream applications such as genotyping, cloning and primer walking. While many purification systems are commercially available, the Agencourt AMPure XP reagent system is a highly efficient, easily automated PCR purification system that delivers superior quality DNA with no salt or dNTP carryover. The AMPure XP reagent system uses an optimized buffer to selectively and reversibly bind PCR amplicons that are 100bp and larger to solid-phase paramagnetic beads. The excess primers, nucleotides, salts and enzymes from the reaction are removed using a simple Bind-Wash-Elute procedure (Figure 1). Requiring no centrifugation or vacuum filtration, the AMPure XP reagent system is highly amenable to automation.
This application note describes the use of the Biomek 4000 Laboratory Automation Workstation to automate PCR reaction setup, as well as amplicon purification using the AMPure XP system. The pipetting tools, updated software and Windows 7 compatibility improve the ease and speed of reaction setup. One to 192 PCR sample reactions can be setup in two 96-well PCR plates using up to two plates of master mixes, two primer sources and two sample sources, including master mix made from individual components. Amplification of a test template was highly efficient and reproducible, with yields equivalent to manual amplification. Efficient and reproducible automated AMPure XP purification can be completed in less than 90 minutes for 192 PCR samples (10 – 70 μL). Both procedures were shown to be free of cross-contamination between wells.
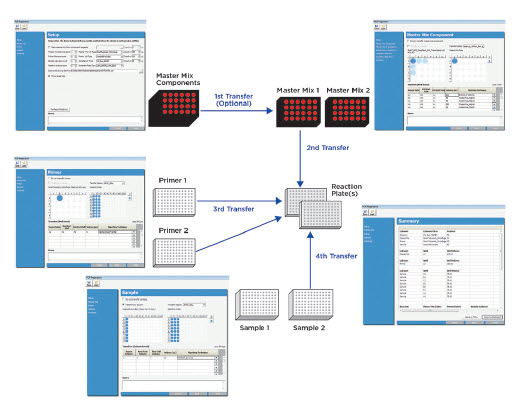
Figure 2. Overview of the automated PCR reaction setup process on the Biomek 4000 Workstation, illustrating the pop-up interface and two 96-well PCR reaction plates that can be set up from different reagent sources.
Materials
Reagent Source
- Agencourt AMPure XP kit, Beckman Coulter, Brea, CA (P/N A63882)
- KAPA SYBR Fast Universal qPCR Kit, KAPA Biosystems, Woburn, MA
- AccuPrime Supermix I, Life Technologies, Carlsbad, CA
- pGEM DNA vector, Promega, Madison, WI
- M13 Forward Primer: 5’-TAA TAC GAC TCA CTA TAG GG-3’, IDT, Coralville, IA
- 1000bp_pGEM Reverse Primer: 5’-TCT AGT GTA GCC GTA GTT AGG-3’, IDT, Coralville, IA
- Human Genomic DNA, Promega, Madison, WI
- β-actin Primer Pairs, Promega, Madison, WI
- PCR Master Mix, Promega, Madison, WI
- 2% agarose gel, Life Technologies, Carlsbad, CA
- Nuclease-free Water, Life Technologies, Carlsbad, CA
- Denatured 70% Ethanol, American Bioanalytical, Natick, MA
Instruments
- Biomek 4000 Laboratory Automation Workstation, Beckman Coulter, Brea, CA (P/N B22638)
- 7900 HT Fast Real-Time PCR System, Life Technologies, Carlsbad, CA
Results and Discussion
Reproducible and Reliable PCR Reaction Setup
The key feature for this application on the Biomek 4000 Workstation is flexibility. With the visual-well-selection user interface, a user can easily set up two 96-well PCR plates for various combinations of master mixes, primers, and samples, as well as create master mixes from single components (Figure 2). This flexibility enables automation of a variety of PCR formats and reaction setup conditions.
Human genomic DNA (24 ng/reaction), β-actin primer pairs and ready-to-use PCR master mix (Promega) were used for automated PCR setup employing default automation transfer techniques. The PCR results showed the absence of the β-actin amplicons (285 bp) in any negative control wells in which human genomic DNA template was replaced by water (Figure 3).
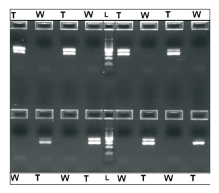
Figure 3. Electrophoretic separation on 2% agarose of amplified human genomic DNA (24 ng per 2 5μL reaction) using β-actin primer pairs and ready-to-use PCR master mix. 20 μL of the reaction volume was loaded onto the gel. The results show no presence of the β-actin amplicons (285bp) in any negative control containing no gDNA template (W), versus robust amplification in adjoining plate wells that contained the human gDNA template (T). The middle lane (L) is a 100bp DNA Ladder.
The Biomek 4000 Workstation was also used to automate setup of real-time amplification of the 285bp Human β-actin gene utilizing a ready-to-use KAPA SYBR qPCR master mix and default automation transfer techniques. The data showed highly consistent amplification with a coefficient of variation (CV) of 1.74% across 32 amplified samples (Figure 4).
Efficient and Reliable Automated AMPure XP Purification
The biggest advantage of automating PCR purification on the Biomek 4000 is speed, as two 96-well PCR plates of samples can be purified in less than 90 minutes for volumes between 10 to 70 μL. The Biomek 4000 AMPure XP method allows users to select the number of samples to be purified, as well as user ID, sample, and reagent lot number tracking via LIMS data collection. Plasmid DNA fragments (1000 bp pGEM) produced by PCR were purified on Biomek 4000 using this method, producing equivalent yield and purity (260nm/230nm ratio) to amplicons purified using the manual process (Table 1).
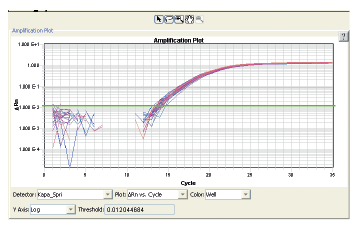
Figure 4. Real-time β-actin PCR reaction results from 32 samples. Each β-actin qPCR reaction amplified 8 ng of human genomic DNA using β-actin primer pairs and KAPA SYBR FAST qPCR Master Mix. The samples gave an average Ct value of 15.72 with 1.74% CV, illustrating highly consistent liquid transfers.
In order to measure cross-contamination during automated purification, the 452bp standard from the KAPA SYBR Fast Universal qPCR Kit was used as template to generate a large volume of PCR fragments. These were then purified using the Biomek 4000 AMPure XP method. The purification was performed on a 96-well plate containing the amplified 452bp fragments in one well and water in the adjacent well (Figure 5A). These purified samples were then transferred to a 384-well real-time PCR plate. Each purified sample (PCR fragment or water) was placed in the upper left corner of a four-well quadrant on the 384-well plate, with nothing added to two of the other wells, and a PCR calibration standard added to the fourth well (Figure 5B).
Real-time amplification was then performed using the ABI7900HT Fast Real-Time PCR system and the KAPA SYBR FAST master mix. True amplification was observed only in the wells containing the 452bp PCR fragments that had been purified using the automated AMPure XP system, and not in the control wells which contained water before purification (Figure 5C). The accuracy of quantitation using the automated dispensing of calibration standards was excellent, resulting in an R2 value of 0.9849. All of the re-purified fragments showed nearly identical amplification curves and Ct values, demonstrating the excellent reproducibility of the automated purification system.
| Automation (Column #1-3) |
Manual (Column #4) |
Automation/ Manual (%) |
|
| Yield (μg/mL) |
5.5823 |
5.8491 |
95.4 |
| Purity (260 nm/230 nm Ratio) |
2.02 |
1.97 | 102.5 |
Table 1 Purification efficiency data using automated AMPure XP. 1000 bp pGEM plasmid DNA PCR fragments were purified using the Biomek 4000 AMPure XP method and a manual process. Yield (95.4%) and purity (102.5%) obtained in triplicate by automation using three columns were compared to those obtained from manual purification.
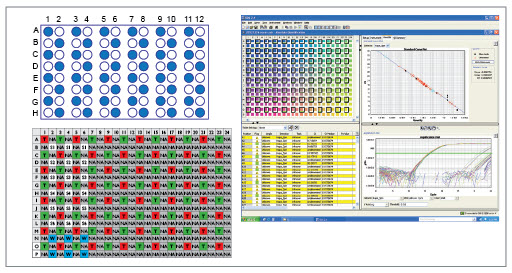
Figure 5. (A) A 96-well plate containing water (blue wells) and 70 μL of 452bp PCR fragments produced by manual amplification of the standard from the KAPA SYBR Fast Universal qPCR Kit (white wells), which was used to demonstrate the automated AMPure XP purification system.
(B) After purification, the samples were transferred to a 384-well real-time PCR plate in such a manner that each sample from A was represented by four wells on the real-time plate: one for the sample, one for a real-time PCR calibration standard, and two that are empty. T= AMPure reagent eluate samples; S = Calibration standards 1-6; W = Water blank controls for the real-time PCR reaction; NA = empty wells. Red wells are negative samples derived from the blue wells containing water in A, and green wells are positive samples derived by purification from the white wells containing 452bp fragments in A.
(C) The results of real-time amplification of the samples in B, illustrating true amplification of only the purified 452bp fragments, and no cross-contamination in samples derived from purification of the wells containing water in A. All of the re-purified fragments showed nearly identical amplification curves and Ct values, demonstrating the excellent reproducibility of the automated purification system.The noise under the amplification curve is derived from primer-dimer amplification.
Conclusion
Automation solutions for both PCR reaction setup and purification can help users from small, medium and high throughput laboratories improve efficiency and results. The Biomek 4000 Workstation and AMPure XP purification system improve reproducibility from day-to-day and across laboratories, reduce the opportunity for human error, and cut labor and reagent costs. The automation templates are simple, fast, and easy to use. The resulting amplification is highly reproducible, and the purification matches the yield and purity of manual procedures, while also delivering highly reproducible results. In addition, both processes are completed without cross-contamination, assuring reliable results.
Biomek Automated Workstations are not intended or validated for use in the diagnosis of disease or other conditions. Beckman Coulter Life Sciences genomic reagent kits are for research use only.
