Enhancing Vaccine Development and Production
Efficient Centrifuge Systems for Vaccine Development and Production
 Author
Author
Chad Schwartz, PhD, Senior Application Scientist
Beckman Coulter, Inc., Life Sciences, Indianapolis, IN USA
The increasing ability of vaccines to impact quality of life is fueling demand for ever-better vaccine products, from new therapeutics for cancer and seasonal influenza, to averting threats of bioterrorism and emerging diseases. Beckman Coulter, Inc. offers a full continuum of centrifuge-related products to enhance vaccine development and production. From initial cell clarification to inactivated viral purification or splitting, Beckman Coulter, Inc. has the perfect centrifuge for your laboratory. Drawing on more than 6 decades of expertise, our full line of centrifugation products delivers quality separations in a short amount of time. Whether you are focused on viral, bacterial, or cell-based vaccines, our centrifuge technology speeds your processes, improves your yield, and allows you to achieve demanding development and production goals. In addition to centrifuges, Beckman Coulter offers complementary solutions for the vaccine environment, including automated cell viability analyzers, flow cytometry solutions, and protein characterization systems. Consider us your partner in creating and delivering high-quality vaccine products from beginning to end.
 Performance
Performance
Our instruments, rotors, tubes, bottles and adapters are designed to work together as a comprehensive system. A modular approach satisfies cross-contamination issues by providing lot-to-lot comparability, which enables faster time-to-market, reduces pharmaceutical development costs and ensures optimum dose efficacy, all with the reliability and long-term commitment you’ve come to expect from Beckman Coulter. Our instruments and rotors are designed with the consumer in mind as researchers want faster speeds, better controlled temperature and vacuum systems, larger capacities, and increased ease of use for quicker, more reliable, higher throughput, and reproducible separations.
Compliance
The traceability and electronic run records of the Avanti JXN and Optima XPN software support 21 CFR Part 11 compliant and GLP/GMP environments. The embedded software tracks a long list of run parameters, usage histories, rotor cycles and more. Please see application note CENT-512APP09.14-A for a more detailed look on how Avanti JXN/Optima XPN centrifuges support 21 CFR Part 11 compliance.
 |
| Workflow screen capture from Optima XPN. |
Flexibility
As a global leader in centrifugation, Beckman Coulter delivers innovative centrifuge solutions that enhance the productivity, efficiency and compliance of vaccine facilities worldwide. Beckman Coulter centrifuge solutions meet your facility’s specific needs throughout the vaccine process, from research and development to production and validation. Beckman’s product line offers an array of optimized centrifuge and rotor systems that allows researchers to select the best fit for their vaccine-related needs. An integrated library of centrifuge instruments, rotors, tubes and accessories enables a high degree of customization and versatility in the use of your Beckman Coulter systems, ensuring efficiency, productivity and safety at each step in the process.
Ease of Use
Beckman Coulter’s intuitive, user-friendly instrument software makes it easy to program centrifuge runs from within the laboratory or even remotely. The Optima XPN/Avanti JXN software provides large digital displays, a comprehensive set of help options, user programmed workflows, and printable run reports, all in a touchscreen format. The software systems for both the Optima XPN and Avanti JXN are consistent with each other, making it easy for customers to go back and forth between instruments. Furthermore, our ergonomic system design enhances operator safety, comfort and productivity, keeping researchers happy and healthy.
 |
| Figure 1.Experimental set up and aliquots of bacteria sample. |
Clean Room Suitability
Beckman Coulter’s BioSafe* centrifuge systems are equipped with a pharmaceutical-grade sterilizing filter system which alleviates concerns over unwanted particles escaping into the vaccine production environment. Our pharmaceutical-grade sterilizing filters are manufactured in a controlled environment and undergo extensive integrity testing. The filter components have met the specifications for biological tests listed in the current revision of the United States Pharmacopeia (USP) for Class VI-121˚C plastics and bacterial retention in conformance with the applicable requirements of the FDA Guideline Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice. Furthermore, the filter system undergoes tests of cleanliness in accordance with Title 21 of the U.S. Code of Federal Regulations (CFR) parts 211.72 and 210.3 (b) (6), oxidizable substances, pH, and pyrogens.
Exemplary Service
Our reputation for excellence, quality and customer service is widely recognized—and is the reason biological facilities around the world look to us for answers to their centrifugation challenges. Our centrifuge technology for vaccine development and production is designed to meet the exacting standards required of the biologics environment. Our technology is backed by Beckman Coulter’s ISO 9001 Certified Field Service Organization. Comprised of factory trained and certified engineers, our service operation has a reputation that is unmatched in the industry. In addition, we offer Installation Qualification and Operational Qualification (IQ/OQ), and our knowledgeable customer service representatives handle your requests promptly to keep your facility running efficiently.
A Powerful Lineup
Beckman Coulter manufactures and supports a long list of high-performance ultracentrifuges; Table 1 is not a comprehensive list, but highlights 4 of Beckman Coulter’s most popular centrifuges for vaccine development and accompanying commonly-used rotors in vaccine production. Many rotors are interchangeable among centrifuge series’. Additionally, Beckman Coulter offers HarvestLine single-use centrifuge bottle liners that provide a significant improvement in the centrifugation of biological material. HarvestLine System liners eliminate time-consuming manual scraping of harvested solids from labware and enhance operator biosafety. HarvestLine liners can be sterilized (gamma irradiation) and frozen to facilitate your production process.
Table 1. Beckman Coulter Centrifuges and Accompanying Rotors for Vaccine Production.
| Optima XPN | Ti-15 Zonal | Max. Speed: 32,000 rpm Capacity: 1,675 mL Max. Flow Rate: 50 mL/min |
Efficient isolation of subcellular particles and antigens |
| SW-32 Ti | Max. Speed: 32,000 rpm Capacity: 231 mL |
Rate-zonal separation of particles | |
| CF-32 Ti Continuous Flow | Max. Speed: 32,000 rpm Capacity: 430 mL Flow Rate: 150 mL/min |
High-throughput isolation of large viruses and bacteria | |
| Type 45 Ti Fixed Angle | Max. Speed: 45,000 rpm Capacity: 564 mL |
Rate-zonal separation of particles | |
| Avanti JXN-26 | JLA-8.1000 Fixed-Angle | Max. Speed: 5,000 rpm Capacity: 6,000 mL |
Large-volume processing of cells, viruses, and precipitates |
| JLA-9.1000 Fixed-Angle | Max. Speed: 9,000 rpm Capacity: 4,000 mL |
Large-volume processing of cells, viruses, and precipitates | |
| JCF-Z Reorienting Gradient | Max. Speed: 20,000 rpm Capacity: 1,750 mL |
Processing of small or large viruses, bacteria, and/or whole cells | |
| JCF-Z Continuous Flow Standard Pellet Core | Max. Speed: 20,000 rpm Capacity: 1,900 mL |
High-throughput isolation and concentration of viruses and bacteria | |
| Avanti J-HC | JS-5.0 Swinging Bucket | Max. Speed: 5,000 rpm Capacity: 9,000 mL |
Cell harvesting with very high throughput |
| J6-MI | JS-4.2 Swinging Bucket | Max. Speed: 4,200 rpm Capacity: 6,000 mL |
Rapid pelleting of large quantities of cells, cell debris, and precipitates |
| JE-5.0 Elutriator | Max. Speed: 5,000 rpm | Gentle, yet powerful technique for harvesting large populations of viable cells |
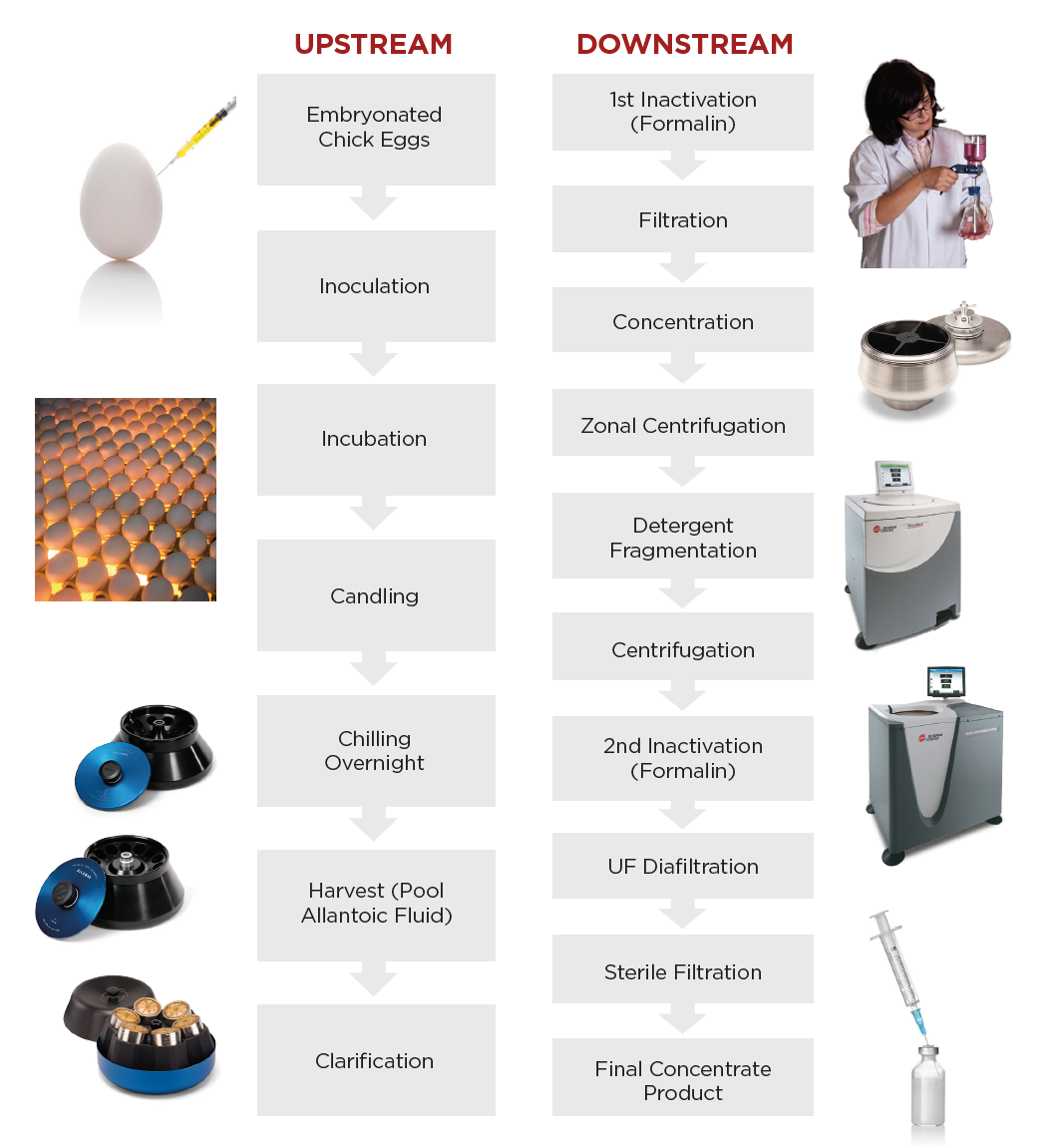 |
| Figure 1. Typical workflow for egg-based influenza vaccine manufacturing.1 |
Vaccine Development Workflow and Beckman Coulter
There are two main types of vaccine development: egg-based and cell-based. Figure 1 demonstrates a typical workflow for egg-based influenza vaccine manufacturing1. The upstream process starts with acquiring embryonated eggs from biosecure flocks, which are subsequently inoculated and incubated for several days to allow the virus to sufficiently multiply. The eggs are then candled to ensure there are no cracks and then chilled overnight. The next day, the allantoic fluid is harvested and clarified by the appropriate means of centrifugation, depending on the required volumes for production. The Avanti JXN-26 is a perfect complement to your workflow in this step, providing ample volume and more than enough speed.
The virus is then inactivated by chemical means, filtered, and concentrated. Zonal centrifugation is now performed to separate the virus from other contaminating particles by size and shape in a sucrose solution. Here, the Optima XPN can be utilized, capable of speeds fast enough to ensure proper separation. The virus is recovered and split by detergent to solubilize the viral membrane, and clarified again by centrifugation to remove large contaminants. Researchers should again take advantage of the Optima XPN in this step. The subunit hemagglutinin and neuraminidase proteins are isolated, inactivated by a second round of formalin, and ultrapurified and concentrated by filtration.
Most egg-based vaccine development protocols call for 3 to 5 centrifugation steps at varying speeds and with different volumes depending on production demands. The protocol lasts around 7 days with further validation, formulation, quality control, and lot release following the purification procedure.
Beckman Coulter centrifuges are compatible with both egg-based and cell-based vaccine development laboratories. Many potential advantages exist with cell-based vaccine development including the lack of dependence on eggs for global production. Additionally, the procedure is more reproducible, standardized, and potentially faster.2,3 The downstream process of cell-based vaccine development is similar to egg-based manufacturing, requiring rounds of centrifugation, inactivation, and filtration. Upstream, the process starts by culturing frozen, preserved cells in an incubator at 37˚C in small volumes. The culture is scaled-up and after reaching a specific density of cells, seed virus is added to the cell-containing bioreactor, such that the virus infects the cell lines and multiplies. The virus is subsequently harvested and clarified by centrifugation.
Vaccine production requires sophisticated instruments performed by cutting-edge researchers. Beckman Coulter is a world leader in innovative centrifuges and here to help your laboratory select the right equipment to generate the most efficient workflow.
CENT-751APP01.15-A

