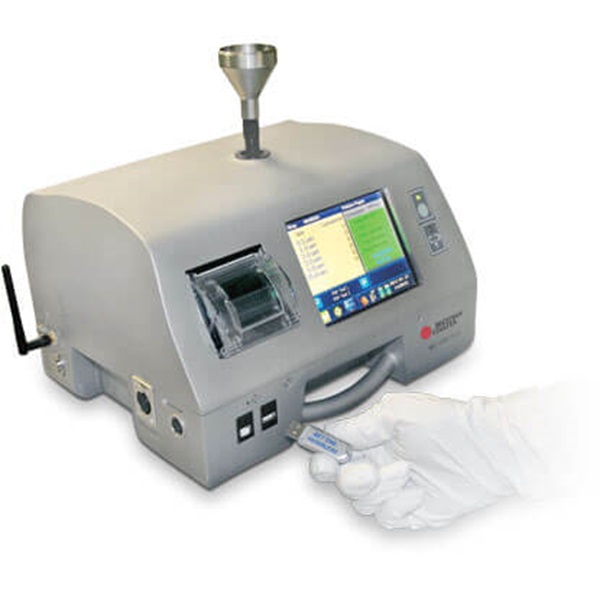
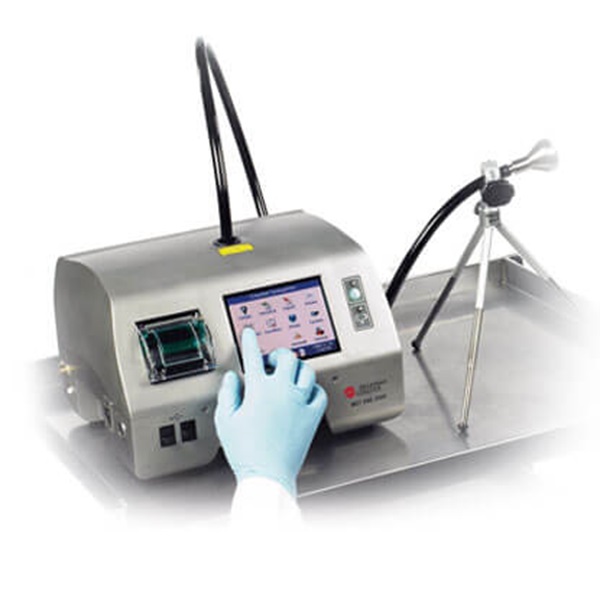
MET ONE 3400 Portable Air Particle Counter
No Longer Available for Purchase
The MET ONE 3400 is no longer available for purchase.
Beckman Coulter Life Sciences will continue to offer routine service and calibration for the legacy family of MET ONE 3400 and 3411 products for a period of five (5) years after July 1, 2021. However, breakdown repairs cannot be guaranteed if the required repair component is made obsolete by our supplier.
Available now
MET ONE 3400+ GMP Cleanroom Routine Environmental Air Particle Counter
The MET ONE 3400+ family has the following advantages over the legacy MET ONE 3400 models:
- Stronger 21 CFR Part 11 Data Integrity features
- Your routine environmental SOP as an interactive map on the counter screen
- Secure electronic records straight from the counter via Wi-Fi and wired Ethernet as standard on all models
- Microsoft Active Directory for User Name and Password control
- More ergonomic design
- Remote access via Web-browser via Wi-Fi and wired Ethernet
- Smaller footprint at just 26.7cm W x 26cm H x 21cm D (10.5” x 10.25” x 8.25”)
- Lighter to carry at just 5.6Kg/12.4lb
Interested in the MET ONE 3400+ air particle counter?
Click to learn more.Explore MET ONE 3400 Series Models
MET ONE 3400 Features
Standards
- ISO 14644-1 enabled sampling and reporting
- ISO 21501-4 unit-to-unit accuracy & reproducibility
- Enables 21 CFR Part 11 and EU Annex 1 compliance
Data Management
- Industry standard connectivity to data management software
- Wireless, Ethernet, serial, and USB communication
- Printed or paperless data output
Operation
- Easy area, group, and location based configuration of operating parameters
- Password protected multi-level access for 21-CFR Part 11-compliant data security
Applications
- Cleanroom routine environmental monitoring
- Aseptic pharmaceutical production
- Continuous environmental monitoring
- Cleanroom classification and validation
- Process troubleshooting
- HEPA filter leak detection