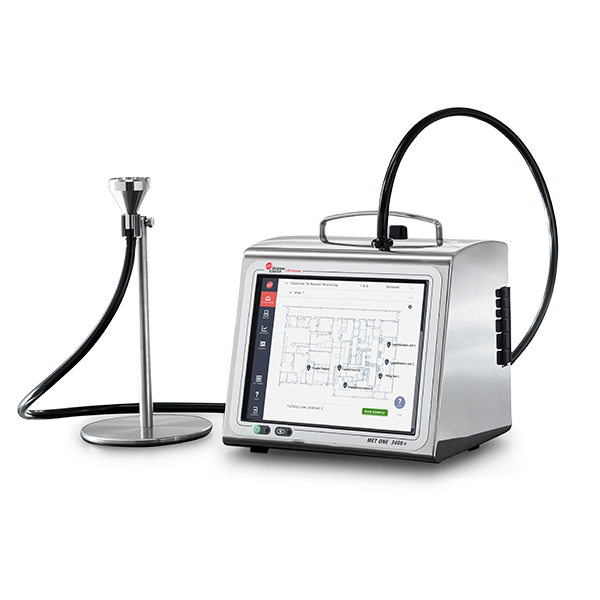
MET ONE 3400+ Series Portable Airborne Particle Counter
Smart Environmental Monitoring for GMP Cleanrooms
The MET ONE 3400+ Series sets a new standard for pharmaceutical cleanroom classification and environmental monitoring. Designed for simplicity, compliance, and efficiency, it transforms complex SOPs into interactive, on-screen workflows—directly on the device. No paper checklists, no separate software, just smarter sampling while supporting ISO 14644 and EU GMP Annex 1 requirements.
Key Features
- Interactive SOP Sampling Maps Upload your routine environmental monitoring SOPs and maps directly into the counter. Users follow step-by-step guidance on the screen, reducing training time and user error.
- Web-Based Remote Access Access the instrument via a standard web browser—no additional software needed. Perform review and approval workflows, manage SOP versions, and export electronic records seamlessly.
- Built-In Audit Trail & Compliance Tools Signatures and access controls help enable 21 CFR Part 11 compliance. Uses Microsoft Active Directory for centralized user authentication and authorization.
- Seamless IT Integration Easy configuration with Microsoft Active Directory. No IT headaches—fast deployment and secure operation within your existing infrastructure.
Benefits
- Reduce Training Time Interactive maps simplify onboarding and daily operation, even for new users.
- Minimize Sampling Errors Guided workflows help ensure consistency and data integrity across all locations.
- Streamline Compliance Supporting 21 CFR Part 11 compliance with secure login, audit trails, and e-signatures—all built in.
- Accelerate Audits and Reporting Quickly locate and filter records during inspections with electronic records.
- No Additional Software Required Everything you need is in the instrument—lower overhead and faster installation and validation.
Explore the Full Potential of the MET ONE 3400+ Series:
- White Paper: GMP Cleanrooms Classification and Routine Environmental Monitoring
- White Paper: MET ONE 3400+ IT Implementation Guide
- Video Series: Understanding the MET ONE 3400+
- Application Note: MET ONE 3400+ LDAP & Active Directory Connection Guide
- Application Note: MET ONE High Pressure Diffuser
- Brochure: GMP Cleanroom Compliance with MET ONE 3400